GBI Biomanufacturing and Allterum Therapeutics Announce Strategic Collaboration to Manufacture Therapeutic Antibody for Clinical Trials
Contact Us For More Information About Our Standalone Drug Product Services >

Experience.
Agility.
Speed.
Quality.
When speed, quality, and flexibility matter for every stage of your biotherapeutics project, choose GBI as your CDMO partner.
Learn MoreGBI is a full-service biopharmaceutical CDMO based in Florida that aims to eliminate manufacturing risk for companies developing complex biologic therapeutics.
Our responsive teams of experts across development, manufacturing, project management, and quality can leverage our many platforms to move your project forward on time and on budget.
With an extensive offering of development services, state-of-the-art manufacturing equipment, and project support, GBI is truly a full-service CDMO biologics partner. Our approach pairs our expert knowledge base with an open mind towards scientific discovery so that together we can develop an optimal way to make our systems work for you and your biologics project, no matter how complex it may be.
Our Single-Source Solution™
GBI’s Single-Source Solution™ offers a wide breadth of integrated CDMO biologics services that are specifically tailored to your needs during manufacturing strategy development. Regardless of your project’s stage, our slate of development and manufacturing services are available to help ensure that your project is completed efficiently and safely.
Explore GBI's Manufacturing Services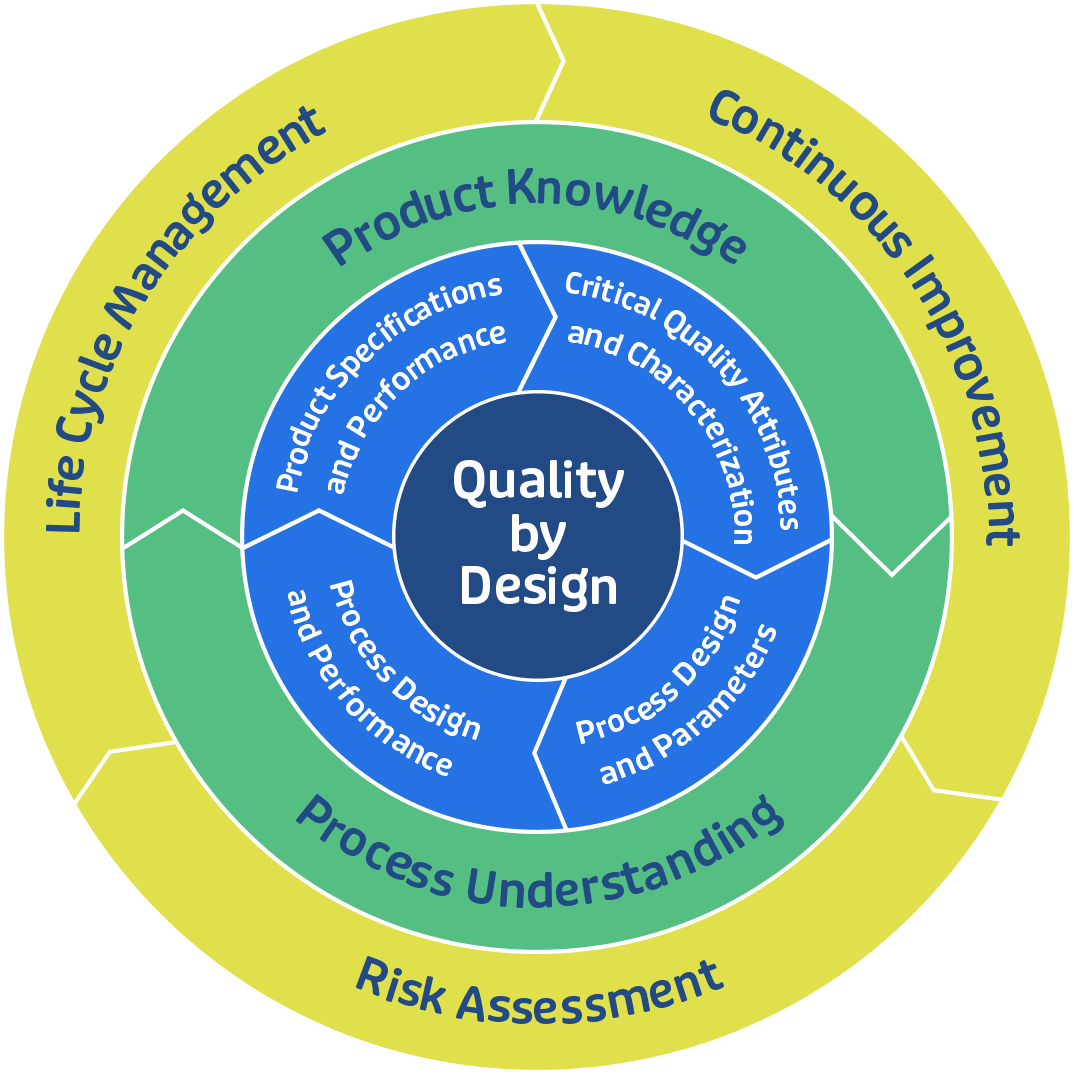
-
Bioconjugation
GBI will help you face the unique challenges of producing and fully characterizing biosimilars.
-
Complex Biologics
GBI is strongly positioned for the growing focus in biopharmaceuticals on the development of complex biologics that allow the targeted delivery of products with multiple functionalities.

Careers
Join GBI’s team of dedicated scientists and other talented professionals in Florida as we expand into a larger facility and a bigger future.







