GBI has been at the forefront of bioconjugation since 2002. Our experience from dozens of projects includes proof of concept through process development, validation and scale up for cGMP manufacturing programs. We are now embarking on multiple commercial programs leaving us well positioned to support clients within the expanding radiopharmaceuticals market, which is expected to exceed $11 billion in value in the next 10 years.
Radiopharmaceuticals have a long history of use in diagnostics and therapies. Currently as a multibillion-dollar business the market is seeing an explosion in development with new therapies, expected to come online every year for the foreseeable future.
There are dozens of developers in diagnostics, theranostics, and therapeutics spearheaded by small virtual and mid-sized integrated developers. Many more institutions and large pharma companies are deeply entrenched in radiopharmaceuticals. Several clinical successes have sparked the interest of larger pharma/biopharma companies, prompting significant M&A activities. Today, the diagnostics segment is the largest portion in the radiopharmaceuticals market, but therapeutic applications are expected to grow faster in the search for better outcomes treating cancer and other diseases.
Technology advancements in nuclear imaging are also fueling development. The need for increased awareness and early diagnosis portend successful treatment of many disorders. Advances in PET and PET/CT facilitate diagnosis in not only cancer but extending to cardiology, infectious diseases, neurology, and immune disorders.
Developing many of these radiopharmaceuticals requires sophisticated partnerships and understanding of chemistry, advanced protein therapeutic development technologies, regulatory pathways and analytical techniques.
This is where GBI expertise facilitates high quality, efficient, and timely drug development in the radiopharmaceuticals space. We are honored to have a strong team with decades of work developing bioconjugation techniques and implementing those techniques into CMC programs. These programs have advanced to manufacturing batches of first-in-human diagnostics, therapeutics and beyond. We’ve worked with numerous isotope additions, for example – Lu-177, Zr-89, Ac-225, Pb-212, In-111.
Since 2002, GBI has performed over 50 bioconjugation projects involving proof of concept, process development, and validation scale-up cGMP manufacturing of multi-gram antibody drugs, theranostic projects, and other protein bioconjugates. This makes GBI a great fit for any project involving the conjugation of monoclonal antibodies or rProteins with other molecules, in particular combining radiopharmaceutical chelators to delivery or other moieties.
Our experience in the field includes a toolbox of techniques with varying targeting moieties, different attachment chemistries, various linkers and linker properties. For example, releasable/non-releasable (cleavable/non-cleavable), assorted payloads including traditional ADC development – coupling small-molecule cancer drugs to antibodies, in which is growing and requires strong development partners.
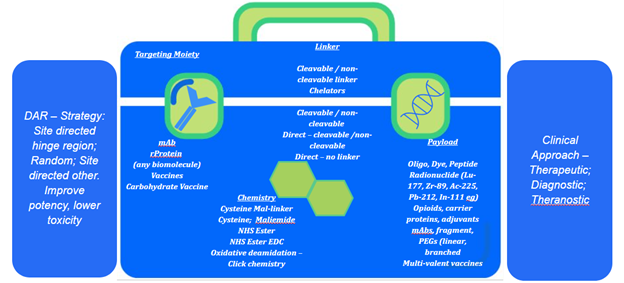
GBI has extensive development experience in other areas of bioconjugation that extend beyond early development into cGMP production for clinical and commercial programs. We have performed conjugation of various molecules to carrier proteins (such as KLH and CRM 197) as immunogens for antibody generation, opioid-conjugate vaccines, conjugating two antibodies, both full-length and fragments. As mentioned for ADCs we’ve applied cleavable and non-cleavable linkers for multiple programs.
As the power of targeting specific cell-types advances, so does the payload or active component evolve. GBI is participating in the development and manufacturing of next-generation complexes by conjugating genomic DNA, RNAs and oligos to antibodies. Historically the idea and value of using nucleic acids as therapeutics has been well demonstrated. However, challenges in targeting the correct cell and then uptake by that cell has limited the advancement for many of those great concepts. This uptake is affected by the size of these molecules and their charge properties. Based on the principals applied to ADCs, GBI helps partners exploit the targeting power of mAbs and mAb-derivatives as they can be applied via advanced bioconjugation knowledge to various nucleic acids to develop next-generation antibody-oligo conjugates (AOCs), in a way that will meet stringent regulatory requirements and as important, scalable, to meet market demands.
Beyond coupling metal chelates onto recombinant proteins and antibodies (including difficult IgMs) for radiolabeling and the growing AOC conjugation techniques, GBI has applied its knowledge and resources to other interesting programs.
For example:
- Adsorption of aluminum hydroxide gels onto antibodies
- Biotinylation of proteins for use with streptavidin- or avidin-based reagents
- Enzyme labeled antibodies for signal generation in diagnostic applications
- Conjugating fluorescent dyes to antibodies
This in addition to developing key properties of therapeutic proteins in the optimization of in vitro and in vivo performance with our extensive background in bioconjugation, our development team has a full in-depth understanding of the issues that accompany these processes. Some of those issues are incorporation levels, active site masking to control the ratio of active drug to delivery moiety (commonly DAR), isotope uptake and stability. All of this to support development activity into cGMP production for FIH trials and later stage production for commercial use to treating patients. GBI expects to be at the forefront of bioconjugation for radiopharmaceutical products as this market continues to accelerate in the quest to deliver life-improving, saving therapies and diagnostic tools.
Need an experienced CDMO in the bioconjugation for radiopharmaceuticals space? GBI is a one-stop shop that can take your project from Drug substance to Drug Product all under one roof! Contact GBI Biomanufacturing today.
