Introduction
Drug product process development is crucial in identifying early risks for the manufacturing process, enabling seamless scale-up from laboratory to commercial production. Effective biologics drug product development leads to optimizing production yield while maintaining high product quality. The biological drug products development and strategies must comply with regulatory requirements, providing the needed data and documentation to support regulatory submissions.
Contract Development and Manufacturing Organizations (CDMOs) provide specialized biological process development services to support a successful drug product development, for scale up production.
Process Development for Drug Products: Key Considerations for Scale-Up

Click HERE to learn more about “DoE for Clone Selection and Process Development to Meet Biosimilar Critical Qualities Attributes and High Productivity”!
Understanding Drug Product Development
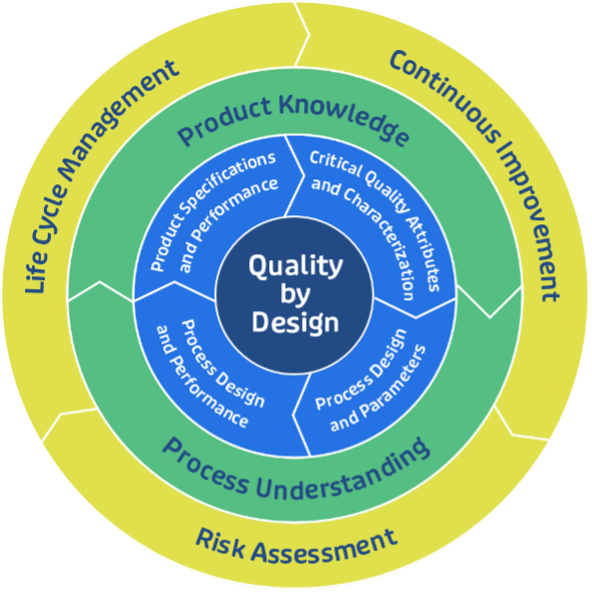
A deep understanding of the drug product development process is necessary to propose any scale-up strategy. It is fundamental to identify the relationships between the product’s critical quality attributes (CQAs), the critical process parameters (CPPs) and critical material attributes (CMAs) that could impact the manufacturing process, as part of the Quality by Design (QbD) approach (see Figure 1).
Identifying CQAs early in the development phase is important to ensuring a smooth transition to commercial production. CQAs, such as potency, purity, and stability, must be monitored closely at each stage of scale-up to ensure product consistency. This is especially important for biosimilar drug product development, where slight variations in production can impact the final product’s safety and efficacy.
Figure 1: Quality By Design Approach
Process Optimization
Scaling up requires optimization of both upstream and downstream processes. The upstream process is focusing on creating the best conditions to grow cells and optimize biological drug output (concentration and quality) in a bioreactor before moving on to purification. During upstream process development key activities to maximize cell growth and product yield are:
- Clone screening and selection.
- Designing the ideal growth/production medium.
- Adjusting bioreactor conditions (such as inductor addition, temperature switch, harvest day, etc.).
The downstream process focuses on biological drug purification and recovery, including chromatographic capture, polishing steps and formulation. Opportunities for optimization to ensure stability, solubility, and delivery characteristics of the drug are:
- Chromatography and resins selection.
- Optimization of buffer conditions, flow rate, and column size.
- Use of bioconjugation to increase efficacy and target specificity.
- Selection of excipients for final formulation.
An optimized, well-designed upstream and downstream lab-scale process is the basis for smooth tech transfer to a cGMP and commercial scale.
Regulatory Considerations
New drug product development is supervised by the Food and Drug Administration (FDA) and European Medicines Agency (EMA). For biologic molecules, it is critical to consider that the process is the product. Antibody structure and function can change by modifying the manufacturing process during development, impacting the drug efficacy and stability. Consequently, the FDA reviews all manufacturing processes and strategies to control the biologic product variations during their evaluation. Per ICH Q8 (R2), biologics drug product development should result in a manufacturing process design with an appropriate control strategy to meet adequate CQAs as defined in a predefined quality target product profile (QTPP). These control strategies are established to help guarantee that manufacturers produce biological products by ensuring consistency and predictability in product performance. It is valuable to start the development process with a vision of the final product.
Scaling parenteral drug product development requires a comprehensive, multi-faceted approach involving technical, regulatory, and market aspects. The success of the transition from small-scale to large-scale production hinges on the development of a robust process that can consistently deliver high-quality, reproducible products.
Considerations and Advancements of CDMO Services for Biologics

Click HERE to read more about “Innovation in Bispecific and Multi-Specific Antibody Engineering”!
Facility Considerations for Biologics Scale-Up
A biological process development facility equipped with advanced technologies is essential for scaling the production of biologics like monoclonal antibodies and biosimilars. After confirming the process and product obtained at lab scale are representative, it is proposed to conduct an engineering run to validate the process at a larger scale, before the GMP and commercial production. This pilot study is an opportunity to focus on process parameters that can affect product quality and resolve any questions about scale-up of the final production.
Working with a process development monoclonal antibodies CDMO such as GBI, that has end-to-end capabilities and technical expertise from process development all the way through to commercial manufacturing, is a significant advantage. The process of scale-up will be more efficient by offering the complete suite of services required from start to end. Successfully transitioning from lab-scale development to commercial production guarantees that the process and product meet safety and efficacy standards.
Innovative Approaches in Drug Product Development Services
Innovative drug product development services offered by CDMOs allow biopharma companies to accelerate timelines and improve scalability. Key strategies in the scaling of biologics include advanced analytics, automation, and integrated solutions for formulation, packaging, and filling.
Emerging Technologies for Process Development
The way drug product process development is scaled has transformed by advances in automation and digitalization. The following innovations have a big impact on process development:
- Data-Driven Process Control by real-time monitoring/analytics with advanced sensors, through process analytical technologies providing immediate feedback and enabling better control over the process.
- Automation systems for cell line development and optimization of process parameters, leading to reduce human error and faster development timelines.
- Artificial Intelligence (AI) and Machine Learning allow the analysis of large datasets for manufacturing processes to propose AI-based models, providing faster process development goals and scaling-up. These AI-models incorporate digital twins providing simulation of results to optimize the process and improve troubleshooting issues before they occur. Recently, a hybrid AI model for process development was successfully validated with GBI data from 20 bioreactors and allowed the selection of the best clone and prediction of the best upstream process conditions to maximize yield and optimize CQAs.
Conclusions
Scaling drug product process development from the laboratory to commercial production requires a combination of technical expertise, robust planning, and a precise infrastructure. A well-equipped CDMO with advanced biological process development services is essential to development success.
During the drug product development, upstream and downstream processes are designed and optimized, with a strong vision of the final product and regulatory requirements in consideration. A CDMO with extensive expertise on process development, end-to-end drug product development services and commercial scale capabilities is fundamental for achieving a reliable and successful biomanufacturing process.
Contact GBI Biomanufacturing Today about Your Clinical & Commercial Manufacturing Needs!

Dr. Bourguignon is Manager of MSAT-USP at GBI. She has over 14 years of experience in biotechnology research and development in upstream processes for biotherapeutic manufacturing, cell-based microfluidic technologies, and toxicity assessment. Dr. Bourguignon is also involved in CDMO project management activities such as technology transfer, project planning, and providing technical support.
